

|
Field/Button |
Status |
Description |
|
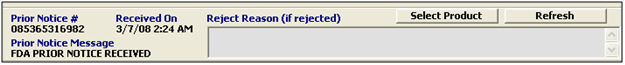
Prior Notice # |
Displayed |
Displays the Prior Notice number, when available. |
|
Received On |
Displayed |
Displays the date and time that this prior notice was received. |
|
Prior Notice Message |
Displayed |
Displays the prior notice message. This will generally show either “FDA PRIOR NOTICE RECEIVED” or “FDA PRIOR NOTICE REJECTED” . |
|
Reject Reason |
Displayed |
Displays the cause of the FDA prior notice reject, if the prior notice is rejected. |
|
Select Product |
Button |
Use to select the product from the product list. This will open the prompt for product code (or you can choose the product from the pick-list). |
|
Refresh |
Button |
Use to refresh the prior notice information section. |
|
Field/Button |
Status |
Description |
|
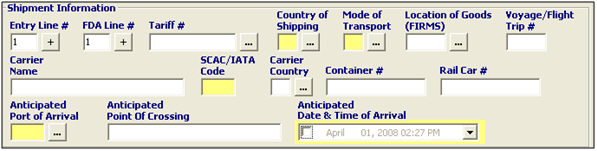
Entry Line # |
Conditional |
Enter the entry line number associated with this FDA prior notice. This is required when the prior notice is associated with an entry number. |
|
FDA Line # |
Mandatory |
Enter the FDA line item number. |
|
Tariff # |
Mandatory |
Enter the tariff number for the commodity. You can also click the ellipsis to bring up the Tariff picker. |
|
Country of Shipping |
Mandatory |
Enter the 2 character country or province code, or choose from the pick-list by clicking the ellipsis. This is the last country from which the product is shipped before arriving in the US, so it may not be the country of origin. |
|
Mode of Transport |
Mandatory |
Enter the 2 digit code for the appropriate mode of transportation of the importing carrier, or click the ellipsis to choose from the pick-list. |
|
Location of Goods (FIRMS) |
Conditional |
Enter the FIRMS code for the arrival location, or choose from the pick-list by clicking the ellipsis. |
|
Voyage/Flight/Trip # |
Conditional |
Enter the voyage number for vessel shipments, flight number for air shipments, or trip number for truck or rail shipments. |
|
Carrier Name |
Conditional |
Enter the name of the importing carrier. This is the carrier that is physically bringing the goods into the US. |
|
SCAC/IATA Code |
Mandatory |
Enter the SCAC or IATA code for the importing carrier. |
|
Carrier Country |
Optional |
Enter the country code for the importing carrier, or choose from the pick-list by clicking the ellipsis. |
|
Container # |
Conditional |
Enter the container number, if applicable. Container numbers are required for container entries (MOT 11, 21, 31, and 41). |
|
Rail Car # |
Conditional |
Enter the rail car number, if applicable. Rail car numbers are required for rail entries (MOT 21/22) |
|
Port of Arrival |
Mandatory |
Enter the port code for the port of arrival, or choose from the pick-list by clicking the ellipsis. |
|
Point of Crossing |
Conditional |
This is the name of the port where the shipment will arrive. This will default if you choose the port code from the pick-list. |
|
Date & Time of Arrival |
Mandatory |
Enter the anticipated date and time of arrival of the shipment, or choose the date from the drop-down menu. This date can be up to 10 days in the future. |
|
Field/Button |
Status |
Description |
|
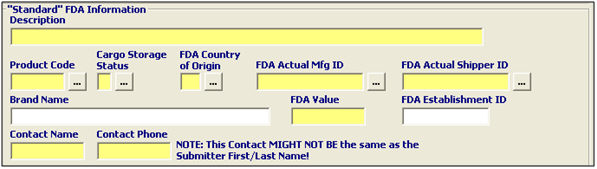
Description |
Mandatory |
Enter a description of the product for FDA purposes. |
|
Product Code |
Mandatory |
Enter the FDA product code for the product, which is the code that identifies the FDA product. Click the ellipsis to use the FDA Product Code Builder. |
|
Cargo Storage Status |
Mandatory |
Enter the code indicating the status of the FDA product. A is used for ambient, F for frozen, and R is for refrigerated. |
|
FDA Country of Origin |
Mandatory |
Enter the FDA country of origin/production, or choose from the pick-list by clicking the ellipsis. Please note that the FDA country of origin may vary from the US customs country of origin or the shipped from country. |
|
FDA Actual Mfg. ID |
Mandatory |
Enter the manufacturer ID of the actual manufacturer, or choose from the pick-list by clicking the ellipsis. |
|
FDA Actual Shipper ID |
Mandatory |
Enter the manufacturer ID of the actual shipper, or choose from the pick-list by clicking the ellipsis. |
|
Brand Name |
Conditional |
Enter the brand name or the distributer of the article. This is required if an Affirmation of Compliance code is transmitted for a radiation emitting device. |
|
FDA Value |
Mandatory |
Enter the value of the product/line, in whole dollars. |
|
FDA Establishment ID (FEI) |
Conditional |
Enter the FDA establishment ID, if necessary. The FEI identifies the final destination for the product. This is not the same as the IRS/Tax ID number. The FEI is a assigned consignee number for the FDA “ship to site”. |
|
Contact Name |
Mandatory |
Enter the name of the party that is completing the prior notice. This is the party that the FDA will contact for information. |
|
Contact Phone |
Mandatory |
Enter the phone number of the party that is completing the prior notice. This is the number where the contact party can be reached for more information. |

|
Field/Button |
Status |
Description |
|
Shipper Registration # |
Conditional |
Enter the FDA Registration number for the shipper. This is the 11 digit number that indicates that the shipper has registered with the FDA. |
|
Manufacturer Registration # |
Conditional |
Enter the FDA Registration number for the manufacturer. This is the 11 digit number that indicates that the manufacturer has registered with the FDA. This field is not required if you are selecting an exemption code. |
|
Mfg Exempt Code |
Conditional |
Enter the manufacturer exemption code, or choose from the pick-list by clicking the ellipsis. This indicates the reason for the manufacturer being exempt from FDA registration requirements. |
|
Producer Firm Type |
Optional |
Enter the manufacturer/processor firm type, or choose from the pick-list by clicking the ellipsis. |
|
Submitter Firm Type |
Mandatory |
Enter the submitter firm type, or choose from the pick-list by clicking the ellipsis. This indicates the type of firm that is submitting the prior notice. |
|
Owner Firm Type |
Mandatory |
Enter the owner firm type, or choose from the pick-list by clicking the ellipsis. |
|
Field/Button |
Status |
Description |
|
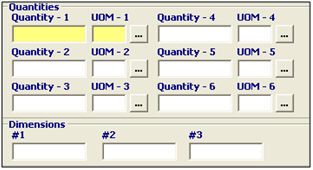
Quantities |
Mandatory |
Enter the quantities associated with the product. This identifies the packaging of the product. You must report each container/package quantity, decreasing from the largest container to the smallest (base unit/quantity). |
|
UOM (Units of Measure) |
Mandatory |
Enter the units of measure associated with the quantities, or choose the UOM from the pick-list by clicking the ellipsis. The smallest must be a base unit of measure. FDA UOM codes may differ from US Customs UOM, so please verify the codes that are being used. |
|
Dimensions |
Conditional |
Enter the product dimensions, when required. For box/cubic dimensions, the order is width, height, and length order. For cylindrical dimensions, the order is diameter and then height. |
|
Field/Button |
Status |
Description |
|
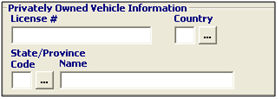
License # |
Conditional |
Enter the license number of the vehicle, if the vehicle is privately owned. |
|
Country |
Conditional |
Enter the country code, or choose from the pick-list by clicking the ellipsis. This is the country in which the vehicle is registered. |
|
State/Province Code |
Conditional |
Enter the state/province code, or choose from the pick-list by clicking the ellipsis. This is the state/province in which the vehicle is registered. |
|
State/Province Name |
Conditional |
Enter the state/province name in which the vehicle is registered. |
|
Field/Button |
Status |
Description |
|
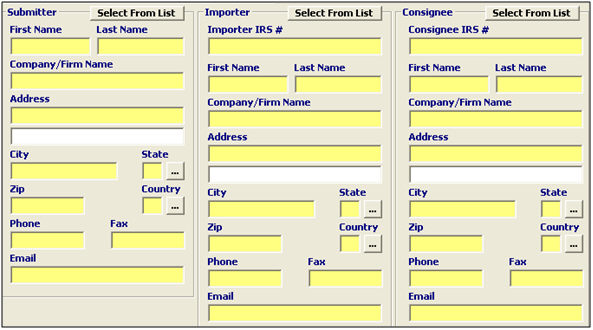
Submitter |
Mandatory |
Enter the name of the person/party with knowledge of the facts surrounding this transaction/shipment. |
|
Importer |
Mandatory |
Enter the name of the importer of record contact for this transaction/shipment. |
|
Consignee |
Mandatory |
Enter the name of the consignee contact for this transaction/shipment. |
|
Select From List |
Button |
Click this button to select the appropriate client from the pick-list. |
|
Name (First/Last) |
Mandatory |
Enter the first and last name for each contact. |
|
Company/Firm Name |
Mandatory |
Enter the company/firm name for each contact. |
|
Address/City/State/ Zip/Country |
Mandatory |
Enter the Address, City, State, Zip code, and Country for each contact. |
|
Phone/Fax/Email |
Mandatory |
Enter the Phone number, Fax number, and email address for each contact. |
|
Field/Button |
Status |
Description |
|
Add |
Button |
Click this button to add a Package/Can code. |
|
Edit/Del |
Buttons |
Click edit to edit the selected package/can code, or click del to delete the code. |
|
Field/Button |
Status |
Description |
|
Package/Can Code |
Optional |
Enter the package/can code for the product on this shipment. |
|
OK/Cancel |
Buttons |
OK will save the package/can code. Cancel will cancel and go back to the main FDA Prior Notice screen without saving. |
When you have completed your Prior Notice Line Item, click OK at the bottom of the FDA prior notice line item data screen. Clicking Cancel will close this screen, without saving.
You can then click Add to add another prior notice line item
Once you’ve completed your Prior Notice data, move on to Completing a Prior Notice.