

This section of the manual contains the instructions for entering FDA into the appropriate PGA screens. It is divided by product type.
We hosted a webinar on entering FDA, please see this link for the recording: https://www.youtube.com/watch?v=YBaio17nLr8
FDA – Disclaim
If your product is flagged for FDA but it does not apply to your product, you can send a disclaimer for FDA.
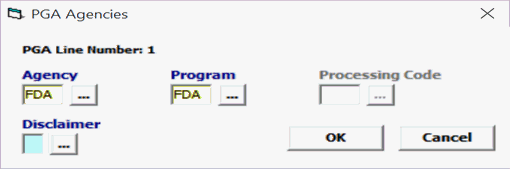
Field/Button |
Status |
Description |
Agency |
Mandatory |
Must be FDA. |
Program |
Mandatory |
FDA |
Processing Code |
Not used |
Not used |
Disclaimer |
Mandatory |
1 Select the appropriate Disclaim Option A = Product is NOT regulated by this Agency B = Data is NOT required per Agency Guidance |
OK/Cancel |
Buttons |
Click OK to save the PGA Information and move on to the next screen. Cancel will cancel and go back to the PGA screen without saving. |
Click "Ok" to return to the Line Items screen.
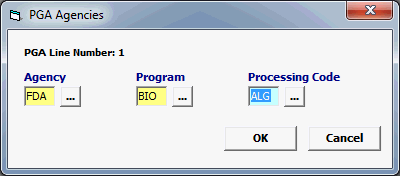
Field/Button |
Status |
Description |
Agency |
Mandatory |
Must be FDA. |
Program |
Mandatory |
BIO |
Processing Code |
Not used |
Enter the appropriate Processing Code (ALG, BBA, BDP, BLD, etc) |
OK/Cancel |
Buttons |
Click OK to save the PGA Information and move on to the next screen. Cancel will cancel and go back to the PGA screen without saving. |
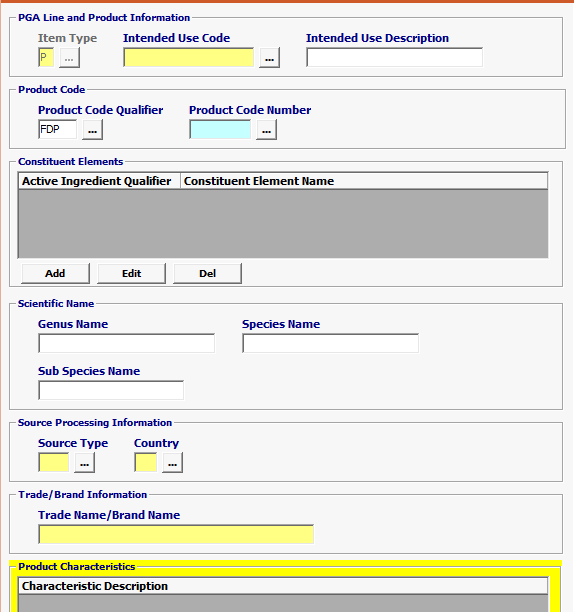
Field/Button |
Status |
Description |
Item Type |
Mandatory |
Defaulted to P for Product. |
| Intended Use Code | Conditional | Enter Intended Use Code *** If the Intended Use is unknown, you have the option to choose UNKN-Unknown but if not required, leave blank. |
| Intended Use Description | Optional | Intended Use Description is optional |
| Product Code Qualifier | Mandatory | “FDP” (FDA Product) |
| Product Code Number | Mandatory | FDA Product Code Must be equal to 7 characters |
| Constituent Elements | Optional | This is an optional PGA input record that provides data pertaining to Constituent Active Ingredient Qualifier, Name of the Constituent Element, Quantity of Constituent Element, Unit of Measure, and Percent of Constituent Element for the product identified by Product Code Number |
| Genus Name | Optional | Scientific Genus Name of the merchandise being entered. |
| Species Name | Optional | Scientific Species Name of the merchandise being entered. |
| Sub Species Name | Optional | Scientific Sub Species Name of the merchandise being entered. |
| Source Type | Mandatory | Mandatory valid value is 39 (Country of Production) or 30 (Country of Source). 294 (Country of Refusal) is MANDATORY if previously refused. |
| Country | Mandatory | Country of production or source is required for Biologics. |
| Trade Name / Brand Name | Conditional | The make of the product (or component) by manufacturer or distributor from the label or invoice. |
| Characteristic Description | Mandatory | Include proper name (if applicable) OR invoice description here - NOT product code description. · *** If the Brand Name is unknown, you have the option to enter Unknown |
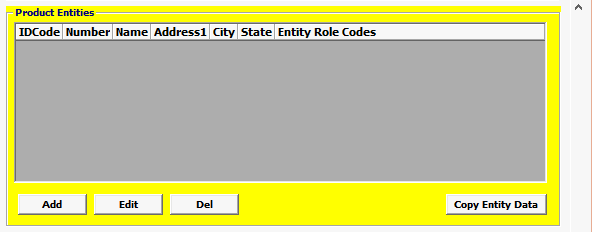
Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the product entities screen. Click Del to delete a line. |
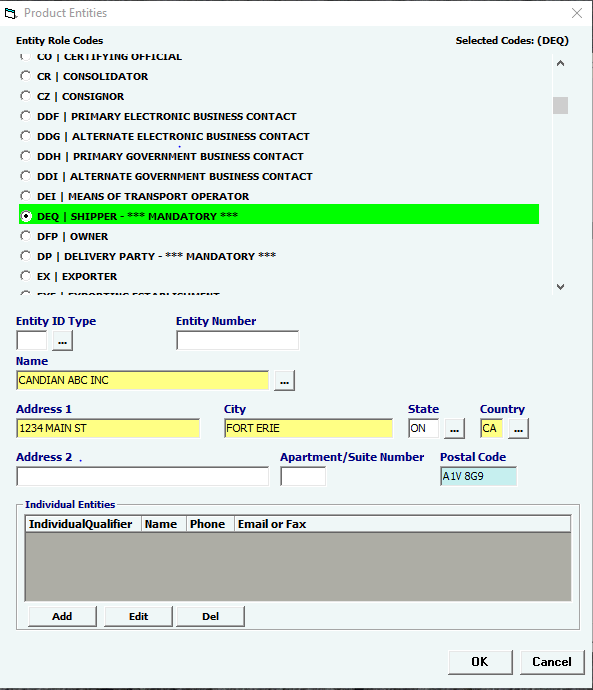
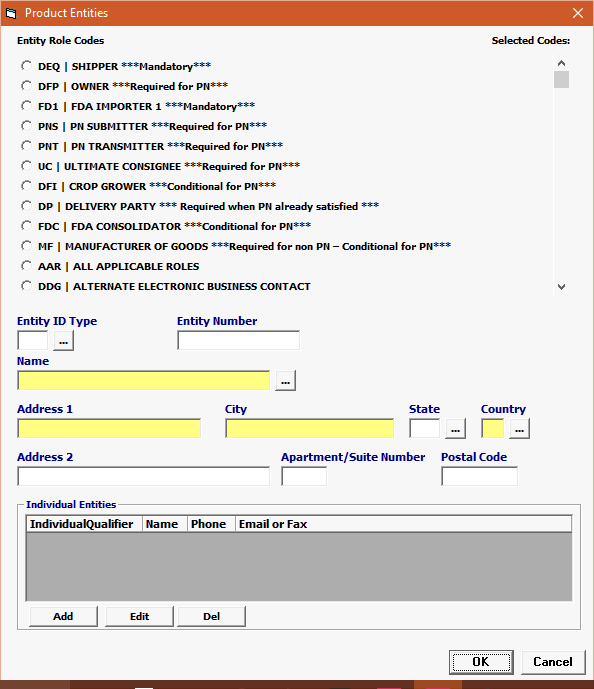
Entity Role Codes |
Mandatory |
Select your Entity Role Codes, one at a time. Under Product Entities, enter all mandatory Entities for BIOLOGICS. (Mandatory entites are marked ***MANDATORY*** in the picklist.) HINT: Mandatory entities for BIOLOGICS MF Manufacturer of goods DEQ Shipper FD1 FDA Importer (Importer of Record) DP Delivered To Party |
Entity ID Type
|
Conditional |
Select an Entity ID Type if you have an Entity ID. |
Entity Number |
Conditional |
MANDATORY Only when indicating EPN (EPA Producer Establishment Number) NOTE, EPN format = NNNNNNAAANNN (N = Number, A = Letter, no dashes). |
Name |
Conditional |
Name of the entity. |
Address 1 |
Conditional |
Address 1 of the entity, mandatory if name is entered. |
City |
Conditional |
City of the entity, mandatory if name is entered. |
State |
Conditional |
State of the entity, mandatory if name is entered. |
Country |
Conditional |
Country of the entity, mandatory if name is entered. |
Address 2 |
Conditional |
Address 2 of the entity. |
Apartment/Suite Number |
Conditional |
Apartment/Suite Number of the entity. |
Postal Code |
Conditional |
Postal code of the entity, mandatory if name is entered. |
Add/Edit/Del |
Button |
Click Add or Edit to open the Individual Entities screen. Each Entity added must have an Individual Entity added. (This is a point of contact) This CAN be the filer’s contact info. Click Del to delete a line. |
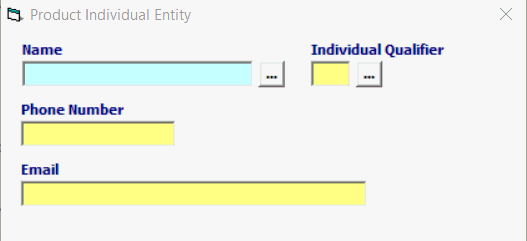
Field/Button |
Status |
Description |
Name |
Conditional |
Name of the individual at the company. MANDATORY for all Roles |
Individual Qualifier |
Conditional |
Individual Qualifier MUST match the Entity Role Code. |
Phone Number |
Conditional |
Phone number of the individual at the company. |
Conditional |
Email of the individual at the company. |
Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the affirmations of compliance screen. Click Del to delete a line. |
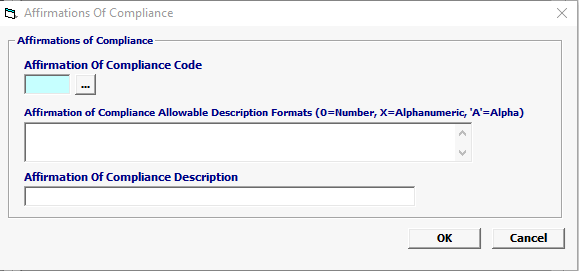
| Affirmation of Compliance Code | Conditional |
A code used to affirm compliance with FDA requirements. |
Affirmation of Compliance Allowable Description Formats |
Conditional |
Text describing the information required by the FDA. This could include a number or a country code, etc. |
Affirmation of Compliance Description |
Conditional |
Enter the Affirmation of Compliance Description |
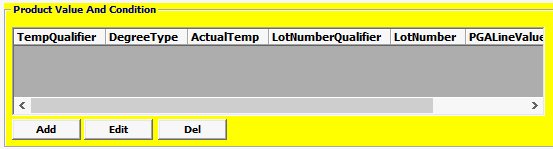
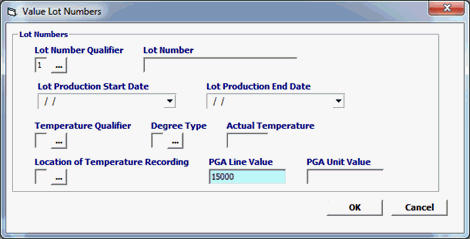
Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the value lot numbers screen. Click Del to delete a line. |
Lot Number Qualifier |
Conditional
|
Code of the entity that assigned the Lot number. For Biologics the only valid value is: 1 = Manufacturer |
Lot Number
|
Conditional |
The lot number that the manufacturer/ producer/grower assigned to the product |
Lot Production Start Date |
Optional |
The date when the production for the Lot started. |
Lot Production End Date |
Optional |
The date when the production for the Lot started. |
Temperature Qualifier |
Optional |
Temperature Category being reported for quality control or preservation purposes. |
Degree Type |
Optional |
F = Fahrenheit, C = Celsius , K = Kelvin |
Actual Temperature |
Optional |
Reported temperature. |
Location of Temperature Recording |
Optional |
Identifies recorded temperature is for A = product B = container C = conveyance |
PGA Line Value |
Mandatory |
The value associated with the PGA line number in whole dollars. I |
PGA Unit Value |
Optional
|
The value of the lowest unit of measure |
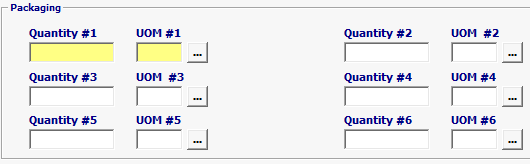
Field/Button |
Status |
Description |
Package Quantity
|
Mandatory |
Outermost (largest=1) packages to the innermost (smallest=6) packages. The total quantity for the packaging level. |
Packaging UOM |
Mandatory |
Type of packaging / packaging level. |
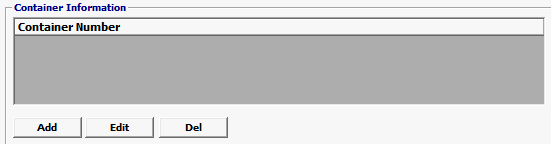
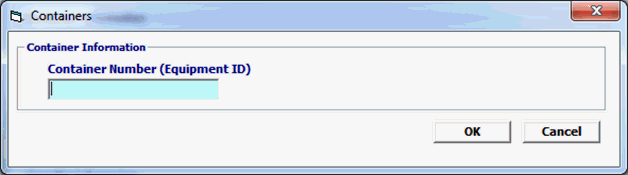
Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the containers screen. Click Del to delete a line. |
Container Number (Equipment ID) |
Optional
|
The number of the shipping container as entered in the Bill of Lading. |
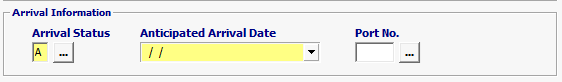
Field/Button |
Status |
Description |
Arrival Status |
Mandatory |
A is the ONLY valid code for FDA: A = Anticipated arrival information. |
Anticipated Arrival date |
Mandatory
|
Date of the anticipated arrival. |
Port No. |
Optional |
Enter a valid port code. |
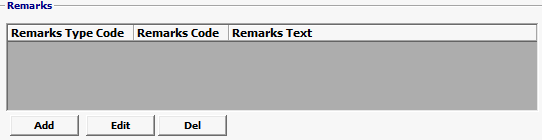
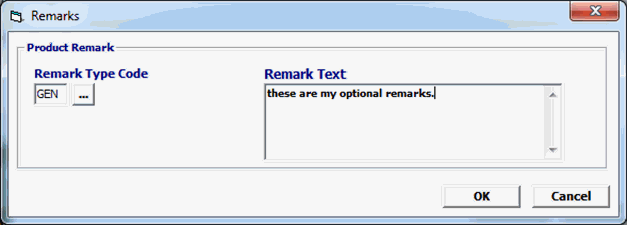
Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the remarks screen. Click Del to delete a line. |
Remarks Type Code |
Optional |
A code indicating the type of remarks. FDA uses either NAM or GEN as its valid values. |
Remarks Text |
Optional |
Free form text relevant to the shipment or the commodity. |
Once you are at the bottom of the PGA screen, click "Ok" to return to the Line Items screen.
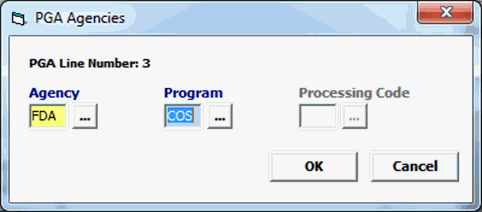
Field/Button |
Status |
Description |
Agency |
Mandatory |
Must be FDA. |
Program |
Mandatory |
COS |
Processing Code |
Not used |
Cosmetics does not require a processing code. |
OK/Cancel |
Buttons |
Click OK to save the PGA Information and move on to the next screen. Cancel will cancel and go back to the PGA screen without saving. |
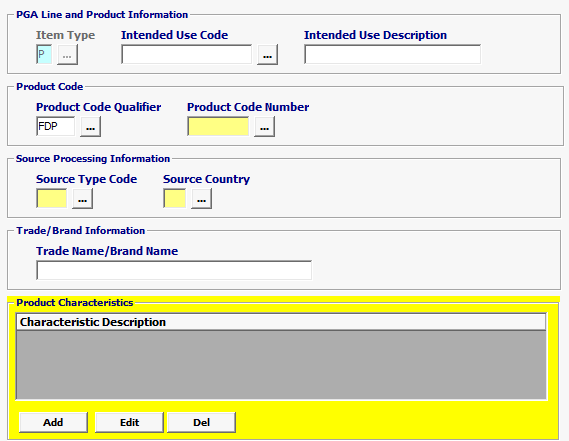
Field/Button |
Status |
Description |
Item Type |
Mandatory |
Defaulted to P for Product. |
| Intended Use Code | Conditional | Enter Intended Use Code *** If the Intended Use is unknown, you have the option to choose UNKN-Unknown but if not required, leave blank. |
| Intended Use Description | Optional | Intended Use Description is optional |
| Product Code Qualifier | Mandatory | “FDP” (FDA Product) |
| Product Code Number | Mandatory | FDA Product Code Must be equal to 7 characters |
| Source Type | Mandatory | Mandatory valid value is 39 (Country of Production) or 30 (Country of Source). 294 (Country of Refusal) is MANDATORY if previously refused. |
| Country | Mandatory | Country of production or source is required for Biologics. |
| Trade Name / Brand Name | Conditional | The make of the product (or component) by manufacturer or distributor from the label or invoice. |
| Characteristic Description | Mandatory | Include proper name (if applicable) OR invoice description here - NOT product code description. · *** If the Brand Name is unknown, you have the option to enter Unknown |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the product entities screen. Click Del to delete a line. |
Entity Role Codes |
Mandatory |
Select your Entity Role Codes, one at a time. Under Product Entities, enter all mandatory Entities for COSMETICS. (Mandatory entites are marked ***MANDATORY*** in the picklist.) HINT: Mandatory entities for COSMETICS MF Manufacturer of goods DEQ Shipper FD1 FDA Importer (Importer of Record) DP Delivered To Party |
Entity ID Type
|
Conditional |
Select an Entity ID Type if you have an Entity ID. |
Entity Number |
Conditional |
Enter the entity number |
Name |
Conditional |
Name of the entity. |
Address 1 |
Conditional |
Address 1 of the entity, mandatory if name is entered. |
City |
Conditional |
City of the entity, mandatory if name is entered. |
State |
Conditional |
State of the entity, mandatory if name is entered. |
Country |
Conditional |
Country of the entity, mandatory if name is entered. |
Address 2 |
Conditional |
Address 2 of the entity. |
Apartment/Suite Number |
Conditional |
Apartment/Suite Number of the entity. |
Postal Code |
Conditional |
Postal code of the entity, mandatory if name is entered. |
Add/Edit/Del |
Button |
Click Add or Edit to open the Individual Entities screen. Each Entity added must have an Individual Entity added. (This is a point of contact) This CAN be the filer’s contact info. Click Del to delete a line. |

Field/Button |
Status |
Description |
Name |
Conditional |
Name of the individual at the company. MANDATORY for all Roles |
Individual Qualifier |
Conditional |
Individual Qualifier MUST match the Entity Role Code. |
Phone Number |
Conditional |
Phone number of the individual at the company. |
Conditional |
Email of the individual at the company. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the affirmations of compliance screen. Click Del to delete a line. |
| Affirmation of Compliance Code | Conditional |
A code used to affirm compliance with FDA requirements. |
Affirmation of Compliance Allowable Description Formats |
Conditional |
Text describing the information required by the FDA. This could include a number or a country code, etc. |
Affirmation of Compliance Description |
Conditional |
Enter the Affirmation of Compliance Description |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the value lot numbers screen. Click Del to delete a line. |
Lot Number Qualifier |
Conditional
|
Code of the entity that assigned the Lot number. For Biologics the only valid value is: 1 = Manufacturer |
Lot Number
|
Conditional |
The lot number that the manufacturer/ producer/grower assigned to the product |
Lot Production Start Date |
Optional |
The date when the production for the Lot started. |
Lot Production End Date |
Optional |
The date when the production for the Lot started. |
Temperature Qualifier |
Optional |
Temperature Category being reported for quality control or preservation purposes. |
Degree Type |
Optional |
F = Fahrenheit, C = Celsius , K = Kelvin |
Actual Temperature |
Optional |
Reported temperature. |
Location of Temperature Recording |
Optional |
Identifies recorded temperature is for A = product B = container C = conveyance |
PGA Line Value |
Mandatory |
The value associated with the PGA line number in whole dollars. |
PGA Unit Value |
Optional
|
The value of the lowest unit of measure |

Field/Button |
Status |
Description |
Package Quantity
|
Mandatory |
Outermost (largest=1) packages to the innermost (smallest=6) packages. The total quantity for the packaging level. |
Packaging UOM |
Mandatory |
Type of packaging / packaging level. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the containers screen. Click Del to delete a line. |
Container Number (Equipment ID) |
Optional
|
The number of the shipping container as entered in the Bill of Lading. |

Field/Button |
Status |
Description |
Arrival Status |
Mandatory |
A is the ONLY valid code for FDA: A = Anticipated arrival information. |
Anticipated Arrival date |
Mandatory
|
Date of the anticipated arrival. |
Port No. |
Optional |
Enter a valid port code. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the remarks screen. Click Del to delete a line. |
Remarks Type Code |
Optional |
A code indicating the type of remarks. FDA uses either NAM or GEN as its valid values. |
Remarks Text |
Optional |
Free form text relevant to the shipment or the commodity. |
Once you are at the bottom of the PGA screen, click "Ok" to return to the Line Items screen.
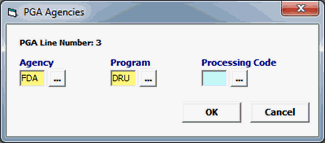
Field/Button |
Status |
Description |
Agency |
Mandatory |
Must be FDA. |
Program |
Mandatory |
DRU |
Processing Code |
Mandatory |
Choose appropriate Processing code (INV, OTC, PHN, PRE, RND) |
OK/Cancel |
Buttons |
Click OK to save the PGA Information and move on to the next screen. Cancel will cancel and go back to the PGA screen without saving. |
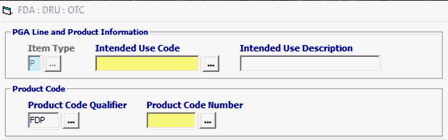
Field/Button |
Status |
Description |
Item Type |
Mandatory |
Defaulted to P for Product. |
| Intended Use Code | Conditional | Enter Intended Use Code *** If the Intended Use is unknown, you have the option to choose UNKN-Unknown but if not required, leave blank.
Prescription (PRE) Finished Form: 080 For Human Medical Use as a Non-Food Product under Controlled Distribution – Prescription (PRE) 970.000 Import for Export 100.000 Importation for Personal Use 155.012 Drug to be used as a constituent part in a Medical Device (Finished Dosage Form Drug) Not Finished Form Active Pharmaceutical Ingredient / Bulk Drug Substance: 150.007 Active Pharmaceutical Ingredient / Bulk Drug Substance for processing into a pharmaceutical product 970.000 Import for Export 150.017 Drug to be used as a component in a Medical Device (Active Pharmaceutical Ingredient / Bulk Drug Substance) Over the Counter (OTC) Finished Form: 130 For Consumer Use as a Non-Food Product – Over the Counter (OTC) 970.000 Import for Export 100.000 Importation for Personal Use 155.012 Drug to be used as a constituent part in a Medical Device (Finished Dosage Form Drug)
Not finished form: Active Pharmaceutical Ingredient / Bulk Drug Substance: 150.007 Active Pharmaceutical Ingredient / Bulk Drug Substance for processing into a pharmaceutical product 970.000 Import for Export 150.017 Drug to be used as a component in a Medical Device (Active Pharmaceutical Ingredient / Bulk Drug Substance)
Pharmaceutical Necessities & Containers (PHN) No intended use codes for this Commodity Sub-Type
Research and Development (INV) 180.009 Chemical for research and development in a pharmaceutical product – clinical trial or other human/animal use
Research and Development (RND) 180.017 Chemical for research and development in a pharmaceutical product – laboratory testing only, no human/animal use |
| Intended Use Description | Optional | Intended Use Description is optional |
| Product Code Qualifier | Mandatory | “FDP” (FDA Product) |
| Product Code Number | Mandatory | FDA Product Code Must be equal to 7 characters |
Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
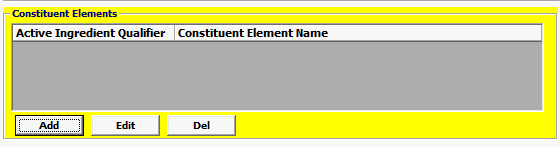
Click Add or Edit to open the constituent elements screen. Click Del to delete a line. |
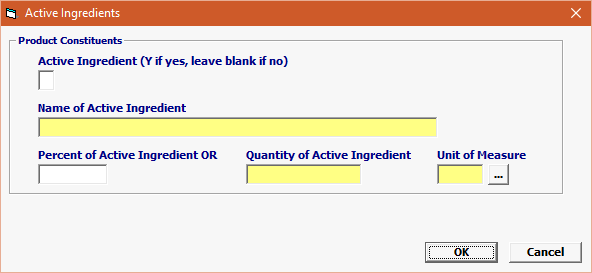
| Active Ingredient | Conditional | If commodity sub-type = PRE, OTC, INV or RND then YES = “Y” if yes, blank (= NO) |
| Name of Active Ingredient | Conditional | IF FINISHED: Name of Active Ingredient contained in the dosage form IF ACTIVE PHARMACEUTICAL INGREDIENT/BULK DRUG SUBSTANCE: name of the Active Pharmaceutical Ingredient (API) |
| Percent of Active Ingredient | Conditional | Only needed for Active Pharmaceutical Ingredients otherwise left blank. |
| Quantity of Active Ingredient | Conditional | IF FINISHED – amount of active ingredient per dose IF API/BULK DRUG SUBSTANCE: total volume of API |
| Unit of Measure | Conditional | IF FINISHED: Unit of measure for Quantity of Constituent Element IF API/BULK DRUG SUBSTANCE: Unit of measure for Quantity of Constituent Element |
Field/Button |
Status |
Description |
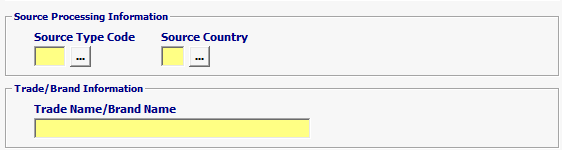
Source Type Code |
Mandatory |
Mandatory valid values are 30 (Country of Source) or 39 (Country of Production). 294 (Country of Refusal) if previously refused. |
| Source Country | Mandatory | Country of production or source is required |
| Trade Name/Brand Name | Conditional | If Government Agency Program Code = ‘DRU’ and [ if PG01 Intended use code = 150.007 (indicates API/Bulk) or Agency Processing Code = ‘PHN’ OR ‘RND’ ] then Trade/Brand Name of the Drug is optional; otherwise, the Trade/Brand Name of the Drug is MANDATORY. |

Field/Button |
Status |
Description |
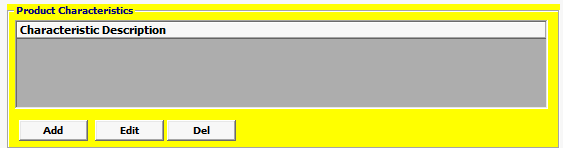
Add/Edit/Del |
Button |
Click Add or Edit to open the product characteristics screen. Click Del to delete a line. |
| Characteristic Description | Mandatory |
This should be Invoice Description NOT product code description. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the product entities screen. Click Del to delete a line. |
Entity Role Codes |
Mandatory |
Select your Entity Role Codes, one at a time. Under Product Entities, enter all mandatory Entities for DRUGS. (Mandatory entites are marked ***MANDATORY*** in the picklist.) For DRU, the mandatory entities are: MF Manufacturer of goods (Final producer for the final drug product). If the product is a bulk API, use “MF” as the Entity Role Code (rather than “GD – Producer of API); If the product is in finished form, provide MF of final product and GD for each API. DEQ Shipper FD1 FDA Importer (Importer of Record) DP Delivered To Party
NOTE: other parties, in addition to those that are Mandatory may be entered *API – Active Pharmaceutical Ingredient *** Rules for Finished Dosage Form Drugs *** IF Intended Use Code = 080 For Human Medical Use as a Non-Food Product under Controlled Distribution – Prescription (PRE) OR 130 For Consumer Use as a Non- Food Product – Over the Counter (OTC) THEN: must include MF and at least one GD |
Entity ID Type
|
Conditional |
Select an Entity ID Type if you have an Entity ID. FDA requires Entity Name and Entity Address. Additionally, FDA prefers to use DUNS numbers for identifying the Entity; IF DUNS is not available THEN FEI |
Entity Number |
Conditional |
Enter the entity number |
Name |
Conditional |
Name of the entity. |
Address 1 |
Conditional |
Address 1 of the entity, mandatory if name is entered. |
City |
Conditional |
City of the entity, mandatory if name is entered. |
State |
Conditional |
State of the entity, mandatory if name is entered. |
Country |
Conditional |
Country of the entity, mandatory if name is entered. |
Address 2 |
Conditional |
Address 2 of the entity. |
Apartment/Suite Number |
Conditional |
Apartment/Suite Number of the entity. |
Postal Code |
Conditional |
Postal code of the entity, mandatory if name is entered. |
Add/Edit/Del |
Button |
Click Add or Edit to open the Individual Entities screen. Each Entity added must have an Individual Entity added. (This is a point of contact) This CAN be the filer’s contact info. Click Del to delete a line. |

Field/Button |
Status |
Description |
Name |
Conditional |
Name of the individual at the company. MANDATORY for all Roles |
Individual Qualifier |
Conditional |
Individual Qualifier MUST match the Entity Role Code. |
Phone Number |
Conditional |
Phone number of the individual at the company. |
Conditional |
Email of the individual at the company. |


Field/Button |
Status |
Description |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Add/Edit/Del |
Button |
Click Add or Edit to open the affirmations of compliance screen. Click Del to delete a line. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affirmation of Compliance Code | Conditional |
A
code used to affirm compliance with FDA requirements. ***
If LST, Device Listing Number is unknown, you have the option
to choose UNKN-Unknown
For Government Program Code = DRU AND Government Processing Code =
THEN Affirmation of Compliance is not required
*** R&D products, Import For Export entries, and Personal Importations do not require AofCs ***
For Government Program Code = DRU AND Intended Use code =
THEN AOC is not required
The list of AoC codes mandatory to FDA Drugs Message Sets is below:
The list of AoC codes conditional to FDA Drugs Message Sets is below:
The list of AoC codes optional to FDA Drugs Message Sets is below:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Affirmation of Compliance Allowable Description Formats |
Conditional |
Text describing the information required by the FDA. This could include a number or a country code, etc. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Affirmation of Compliance Description |
Conditional |
Enter the Affirmation of Compliance Description |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the value lot numbers screen. Click Del to delete a line. |
Lot Number Qualifier |
Conditional
|
Code of the entity that assigned the Lot number. |
Lot Number
|
Conditional |
The lot number that the manufacturer/ producer/grower assigned to the product |
Lot Production Start Date |
Optional |
The date when the production for the Lot started. |
Lot Production End Date |
Optional |
The date when the production for the Lot started. |
Temperature Qualifier |
Optional |
Temperature Category being reported for quality control or preservation purposes. |
Degree Type |
Optional |
F = Fahrenheit, C = Celsius , K = Kelvin |
Actual Temperature |
Optional |
Reported temperature. |
Location of Temperature Recording |
Optional |
Identifies recorded temperature is for A = product B = container C = conveyance |
PGA Line Value |
Mandatory |
The value associated with the PGA line number in whole dollars. |
PGA Unit Value |
Optional
|
The value of the lowest unit of measure |

Field/Button |
Status |
Description |
Package Quantity
|
Mandatory |
Outermost (largest=1) packages to the innermost (smallest=6) packages. The total quantity for the packaging level. |
Packaging UOM |
Mandatory |
Type of packaging / packaging level. |

Field/Button |
Status |
Description |
Arrival Status |
Mandatory |
A is the ONLY valid code for FDA: A = Anticipated arrival information. |
Anticipated Arrival date |
Mandatory
|
Date of the anticipated arrival. |
Port No. |
Optional |
Enter a valid port code. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the remarks screen. Click Del to delete a line. |
Remarks Type Code |
Optional |
A code indicating the type of remarks. FDA uses either NAM or GEN as its valid values. |
Remarks Text |
Optional |
Free form text relevant to the shipment or the commodity. |
Once you are at the bottom of the PGA screen, click "Ok" to return to the Line Items screen.
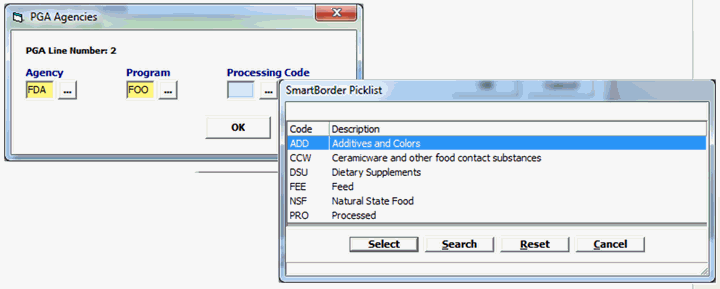
Field/Button |
Status |
Description |
Agency |
Mandatory |
Must be FDA. |
Program |
Mandatory |
FOO |
Processing Code |
Mandatory |
Choose appropriate Processing code (ADD, CCW, DSU, FEE, NSF, PRO) |
OK/Cancel |
Buttons |
Click OK to save the PGA Information and move on to the next screen. Cancel will cancel and go back to the PGA screen without saving. |
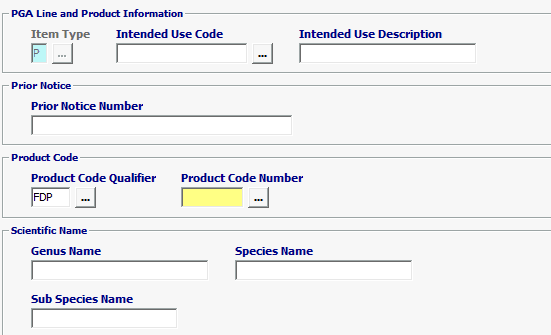
Field/Button |
Status |
Description |
Item Type |
Mandatory |
Defaulted to P for Product. |
| Intended Use Code | Optional |
Enter Intended Use Code *** If the Intended Use is unknown, you have the option to choose UNKN-Unknown but if not required, leave blank.
|
| Intended Use Description | Optional | Intended Use Description is optional |
| Prior Notice Number | Conditional | Enter Prior Notice Number if Prior Notice was previously submitted. |
| Product Code Qualifier | Mandatory | “FDP” (FDA Product) |
| Product Code Number | Mandatory | FDA Product Code Must be equal to 7 characters |
| Genus Name | Optional | Scientific Genus Name of the merchandise being entered. |
| Species Name | Optional | Scientific Species Name of the merchandise being entered. |
| Sub Species Name | Optional | Scientific Sub Species Name of the merchandise being entered. |
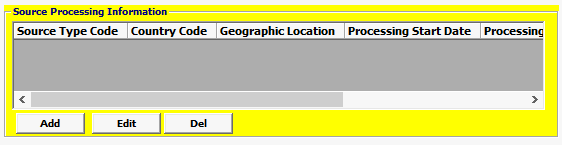
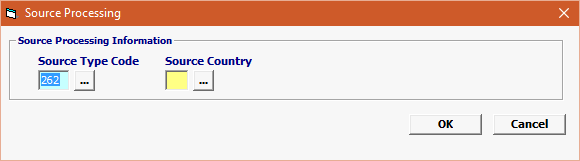
Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the source processing screen. Click Del to delete a line. |
Source Type Code |
Mandatory |
Source Type Code must be selected using the following logic. 1) IF Government Agency Program Code = FOO and Government Agency Processing Code = NSF then use 262 (Place of growth) ELSE Source Type Code can be 39 (Country of Production). IF Government Agency Program Code = FOO, THEN Country of Shipment CSH is required. IF Government Agency Program Code = FOO then Country of Entry Refusal 294 is required, if previously refused from other country(s) |
| Source Country | Mandatory | Foods require the harvesting or production location of the product. |
| Trade Name/Brand Name | Conditional | If Government Agency Program Code = ‘DRU’ and [ if PG01 Intended use code = 150.007 (indicates API/Bulk) or Agency Processing Code = ‘PHN’ OR ‘RND’ ] then Trade/Brand Name of the Drug is optional; otherwise, the Trade/Brand Name of the Drug is MANDATORY. |
Field/Button |
Status |
Description |
| Trade Name/Brand Name | Optional | Market, Trade, or Brand Name that describes the food or feed product at each line level |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the product characteristics screen. Click Del to delete a line. |
| Characteristic Description | Mandatory |
Common, market or usual name or description, NOT product code description of the product. Free form description of the item. |

Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the product entities screen. Click Del to delete a line. |
Entity Role Codes |
Mandatory |
Select your Entity Role Codes, one at a time. Under Product Entities, enter all mandatory Entities for FOOD. (Mandatory entites are marked ***MANDATORY*** in the picklist.)
NOTE: Entities for entries with previously reported Prior Notice (PNSI), the mandatory entities are: MF Manufacturer of goods DEQ Shipper FD1 FDA Importer (Importer of Record) DP Delivered To Party
** NOTE: to submit PRIOR NOTICE, be sure to provide the additional Entities required to satisfy BTA. ** NOTE: Entities for entries with Prior Notice and without the PNSI, the mandatory entities are: DEQ Shipper DFP Owner FD1 FDA Importer 1 PNS PN Submitter PNT PN Transmitter UC Ultimate Consignee
NOTE: Entities for entries with Prior Notice and without the PNSI, which may be required entities are:
MF Manufacturer of goods FDC Consolidator DFI Crop Grower |
Entity ID Type
|
Conditional |
Select an Entity ID Type if you have an Entity ID. FDA requires Entity Name and Entity Address. Additionally, FDA prefers to use DUNS numbers for identifying the Entity; IF DUNS is not available THEN FEI |
Entity Number |
Conditional |
Enter the entity number |
Name |
Conditional |
Name of the entity. |
Address 1 |
Conditional |
Address 1 of the entity, mandatory if name is entered. |
City |
Conditional |
City of the entity, mandatory if name is entered. |
State |
Conditional |
State of the entity, mandatory if name is entered. |
Country |
Conditional |
Country of the entity, mandatory if name is entered. |
Address 2 |
Conditional |
Address 2 of the entity. |
Apartment/Suite Number |
Conditional |
Apartment/Suite Number of the entity. |
Postal Code |
Conditional |
Postal code of the entity, mandatory if name is entered. |
Add/Edit/Del |
Button |
Click Add or Edit to open the Individual Entities screen. Each Entity added must have an Individual Entity added. (This is a point of contact) This CAN be the filer’s contact info. Click Del to delete a line. |

Field/Button |
Status |
Description |
Name |
Conditional |
Name of the individual at the company. MANDATORY for all Roles |
Individual Qualifier |
Conditional |
Individual Qualifier MUST match the Entity Role Code. |
Phone Number |
Conditional |
Phone number of the individual at the company. |
Conditional |
Email of the individual at the company. |


Field/Button |
Status |
Description |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Add/Edit/Del |
Button |
Click Add or Edit to open the affirmations of compliance screen. Click Del to delete a line. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affirmation of Compliance Code | Conditional |
A
code used to affirm compliance with FDA requirements.
The below information may be helpful in regards to optional and conditional Affirmation of Compliance codes:
. The list of optional AoC codes for the FDA food Prior Notice Message Set is below:
The list of conditional AoC codes for the FDA Food (Non-PN) Message Set is below:
The list of optional AoC codes for the FDA Food (Non-PN) Message Set is below:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Affirmation of Compliance Allowable Description Formats |
Conditional |
Text describing the information required by the FDA. This could include a number or a country code, etc. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Affirmation of Compliance Description |
Conditional |
Enter the Affirmation of Compliance Description | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the value lot numbers screen. Click Del to delete a line. |
Lot Number Qualifier |
Conditional
|
Note – for Lot Number Qualifier, if processing code is NSF, Lot Number Qualifier must be 3. Otherwise Lot Number Qualifier is = 1. (per PGA Supplemental Guide 2.4) |
Lot Number
|
Conditional |
The lot number that the manufacturer/ producer/grower assigned to the product |
Lot Production Start Date |
Optional |
The date when the production for the Lot started. |
Lot Production End Date |
Optional |
The date when the production for the Lot started. |
Temperature Qualifier |
Optional |
Temperature Category being reported for quality control or preservation purposes. |
Degree Type |
Optional |
F = Fahrenheit, C = Celsius , K = Kelvin |
Actual Temperature |
Optional |
Reported temperature. |
Location of Temperature Recording |
Optional |
Identifies recorded temperature is for A = product B = container C = conveyance |
PGA Line Value |
Mandatory |
The value associated with the PGA line number in whole dollars. |
PGA Unit Value |
Optional
|
The value of the lowest unit of measure |

Field/Button |
Status |
Description |
Package Quantity
|
Mandatory |
Outermost (largest=1) packages to the innermost (smallest=6) packages. The total quantity for the packaging level. |
Packaging UOM |
Mandatory |
Type of packaging / packaging level. |

Field/Button |
Status |
Description |
Arrival Status |
Mandatory |
A is the ONLY valid code for FDA: A = Anticipated arrival information. |
Anticipated Arrival date |
Mandatory
|
Date of the anticipated arrival. |
Port No. |
Optional |
Enter a valid port code. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the containers screen. Click Del to delete a line. |
Container Number (Equipment ID) |
Optional
|
The number of the shipping container as entered in the Bill of Lading. |
Field/Button |
Status |
Description |
Can Dimensions #1/ #2/ #3 |
Conditional |
*NOTE-If container is a rectangle the dimension should be entered in WIDTH, HEIGHT, LENGTH order. If Cylindrical, the dimensions are in DIAMETER and HEIGHT order. LACF and Acidified: Industry Codes: 02-39, 41, 71, & 72 With Process Indicator Code (PIC): Acidified-I, Aseptic-F, Commercially Sterile-E If the product is LACF, the can measurements (height and diameter) must be provided in CAN DIMENSIONS section unless both the Affirmation of Compliance codes FCE (Food Canning Establishment number) and SID (Schedule Identifier Number) are provided. If Acidified Food (AF), one of the following must be provided unless FCE and SID are provided:
|


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the remarks screen. Click Del to delete a line. |
Remarks Type Code |
Optional |
A code indicating the type of remarks. FDA uses either NAM or GEN as its valid values. |
Remarks Text |
Optional |
Free form text relevant to the shipment or the commodity. |
Once you are at the bottom of the PGA screen, click "Ok" to return to the Line Items screen.
Field/Button |
Status |
Description |
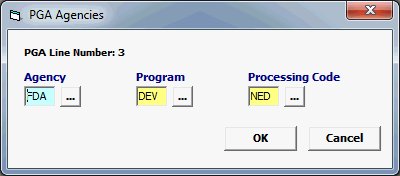
Agency |
Mandatory |
Must be FDA. |
Program |
Mandatory |
DEV |
Processing Code |
Mandatory |
Enter the Applicable Processing code (NED, or RED) |
OK/Cancel |
Buttons |
Click OK to save the PGA Information and move on to the next screen. Cancel will cancel and go back to the PGA screen without saving. |
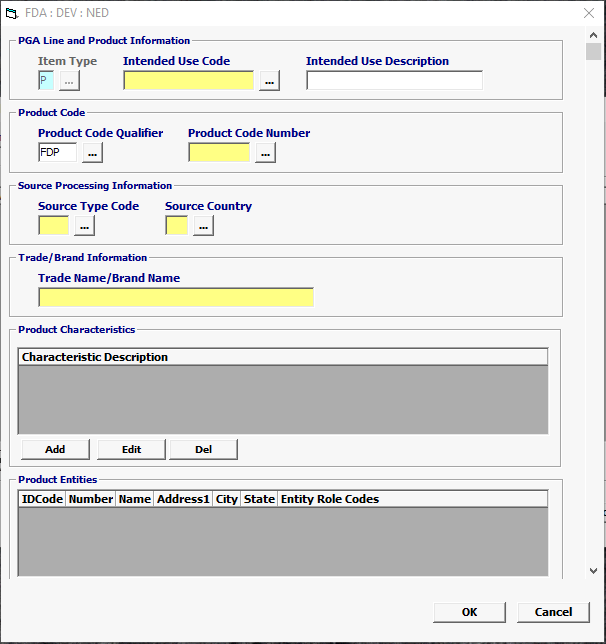
Field/Button |
Status |
Description |
Item Type |
Mandatory |
Defaulted to P for Product. |
| Intended Use Code | Conditional | Enter Intended Use Code *** If the Intended Use is unknown, you have the option to choose UNKN-Unknown but if not required, leave blank. |
| Intended Use Description | Optional | Intended Use Description is optional |
| Product Code Qualifier | Mandatory | “FDP” (FDA Product) |
| Product Code Number | Mandatory | FDA Product Code Must be equal to 7 characters |
| Source Type | Mandatory | Mandatory valid value is 39 (Country of Production) or 30 (Country of Source). 294 (Country of Refusal) is MANDATORY if previously refused. |
| Country | Mandatory | Country of production or source is required for Medical Devices. |
| Trade Name / Brand Name | Mandatory | Trade/Brand Name of the Medical Device. For example, Zimmer Reusable Tourniquet Cuff. |
| Characteristic Description | Mandatory | Free form description, NOT product code description, of the item, either to supplement the above data elements or in place of the above. |

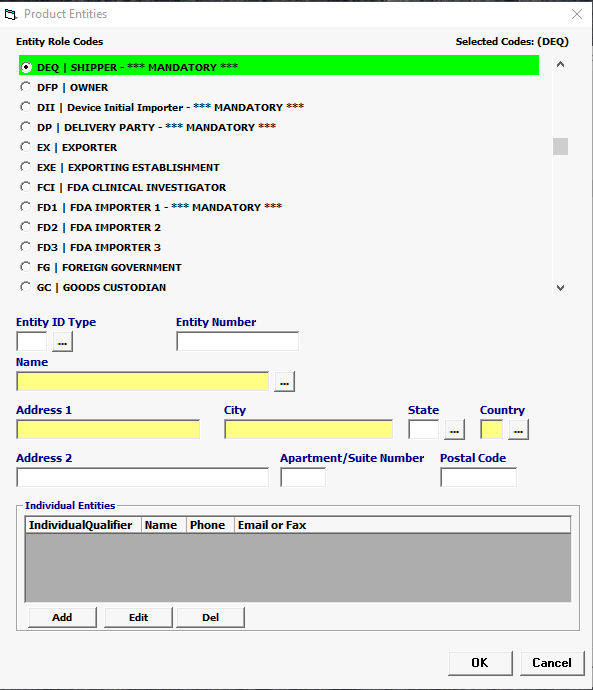
Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the product entities screen. Click Del to delete a line. |
Entity Role Codes |
Mandatory |
Select your Entity Role Codes, one at a time. Under Product Entities, enter all mandatory Entities for MEDICAL DEVICES (Mandatory entites are marked ***MANDATORY*** in the picklist.) HINT: Mandatory entities for MEDICAL DEVICES MF Manufacturer of goods DEQ Shipper FD1 FDA Importer (Importer of Record) DDI Device Initial Importer DP Delivered To Party |
Entity ID Type
|
Conditional |
Select an Entity ID Type if you have an Entity ID. For MEDICAL DEVICES the vast majority of registration numbers are FEIs |
Entity Number |
Conditional |
Enter the entity number |
Name |
Conditional |
Name of the entity. |
Address 1 |
Conditional |
Address 1 of the entity, mandatory if name is entered. |
City |
Conditional |
City of the entity, mandatory if name is entered. |
State |
Conditional |
State of the entity, mandatory if name is entered. |
Country |
Conditional |
Country of the entity, mandatory if name is entered. |
Address 2 |
Conditional |
Address 2 of the entity. |
Apartment/Suite Number |
Conditional |
Apartment/Suite Number of the entity. |
Postal Code |
Conditional |
Postal code of the entity, mandatory if name is entered. |
Add/Edit/Del |
Button |
Click Add or Edit to open the Individual Entities screen. Each Entity added must have an Individual Entity added. (This is a point of contact) This CAN be the filer’s contact info. Click Del to delete a line. |

Field/Button |
Status |
Description |
Name |
Conditional |
Name of the individual at the company. MANDATORY for all Roles |
Individual Qualifier |
Conditional |
Individual Qualifier MUST match the Entity Role Code. |
Phone Number |
Conditional |
Phone number of the individual at the company. |
Conditional |
Email of the individual at the company. |


Field/Button |
Status |
Description |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Add/Edit/Del |
Button |
Click Add or Edit to open the affirmations of compliance screen. Click Del to delete a line. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affirmation of Compliance Code | Conditional |
A
code used to affirm compliance with FDA requirements.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Affirmation of Compliance Allowable Description Formats |
Conditional |
Text describing the information required by the FDA. This could include a number or a country code, etc. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Affirmation of Compliance Description |
Conditional |
Enter the Affirmation of Compliance Description |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the value lot numbers screen. Click Del to delete a line. |
Lot Number Qualifier |
Conditional
|
Code of the entity that assigned the Lot number. For Biologics the only valid value is: 1 = Manufacturer |
Lot Number
|
Conditional |
The lot number that the manufacturer/ producer/grower assigned to the product |
Lot Production Start Date |
Optional |
The date when the production for the Lot started. |
Lot Production End Date |
Optional |
The date when the production for the Lot started. |
Temperature Qualifier |
Optional |
Temperature Category being reported for quality control or preservation purposes. |
Degree Type |
Optional |
F = Fahrenheit, C = Celsius , K = Kelvin |
Actual Temperature |
Optional |
Reported temperature. |
Location of Temperature Recording |
Optional |
Identifies recorded temperature is for A = product B = container C = conveyance |
PGA Line Value |
Mandatory |
The value associated with the PGA line number in whole dollars. |
PGA Unit Value |
Optional
|
The value of the lowest unit of measure |

Field/Button |
Status |
Description |
Package Quantity
|
Mandatory |
Outermost (largest=1) packages to the innermost (smallest=6) packages. The total quantity for the packaging level. |
Packaging UOM |
Mandatory |
Type of packaging / packaging level. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the containers screen. Click Del to delete a line. |
Container Number (Equipment ID) |
Optional
|
The number of the shipping container as entered in the Bill of Lading. |

Field/Button |
Status |
Description |
Arrival Status |
Mandatory |
A is the ONLY valid code for FDA: A = Anticipated arrival information. |
Anticipated Arrival date |
Mandatory
|
Date of the anticipated arrival. |
Port No. |
Optional |
Enter a valid port code. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the remarks screen. Click Del to delete a line. |
Remarks Type Code |
Optional |
A code indicating the type of remarks. FDA uses either NAM or GEN as its valid values. |
Remarks Text |
Optional |
Free form text relevant to the shipment or the commodity. |
Once you are at the bottom of the PGA screen, click "Ok" to return to the Line Items screen.
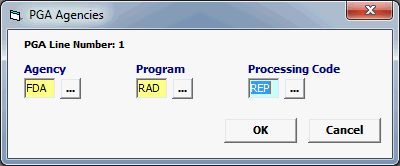
Field/Button |
Status |
Description |
Agency |
Mandatory |
Must be FDA. |
Program |
Mandatory |
RAD |
Processing Code |
Mandatory |
REP |
OK/Cancel |
Buttons |
Click OK to save the PGA Information and move on to the next screen. Cancel will cancel and go back to the PGA screen without saving. |
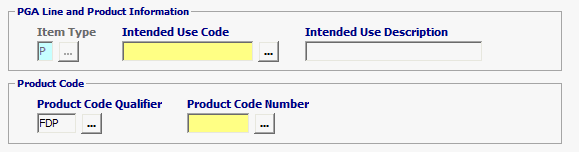
Field/Button |
Status |
Description |
Item Type |
Mandatory |
Defaulted to P for Product. |
| Intended Use Code | Optional |
Enter Intended Use Code *** If the Intended Use is unknown, you have the option to choose UNKN-Unknown but if not required, leave blank.
|
| Intended Use Description | Optional | Intended Use Description is optional |
| Prior Notice Number | Conditional | Enter Prior Notice Number if Prior Notice was previously submitted. |
| Product Code Qualifier | Mandatory | “FDP” (FDA Product) |
| Product Code Number | Mandatory | FDA Product Code Must be equal to 7 characters |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the source processing screen. Click Del to delete a line. |
Source Type Code |
Mandatory |
Source Type Code must be selected using the following logic. 1) IF Government Agency Program Code = FOO and Government Agency Processing Code = NSF then use 262 (Place of growth) ELSE Source Type Code can be 39 (Country of Production). IF Government Agency Program Code = FOO, THEN Country of Shipment CSH is required. IF Government Agency Program Code = FOO then Country of Entry Refusal 294 is required, if previously refused from other country(s) |
| Source Country | Mandatory | A two-letter code that identifies the country from where the product was produced, packed or shipped. |
Field/Button |
Status |
Description |
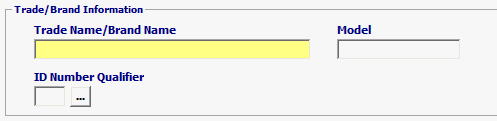
| Trade Name/Brand Name | Mandatory | Trade/Brand Name of the Radiation-Emitting Device. For example, Sony Laser scanner RM2. |
| Model | Optional | The Item Identity Number. This is the Model Number or the Serial Number based on the qualifier entered below |
| ID Number Qualifier | Optional | Code identifying the type of Item Identity Number being provided |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the product characteristics screen. Click Del to delete a line. |
| Characteristic Description | Mandatory |
Common, market or usual name or description, NOT product code description of the product. Free form description of the item. |

Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
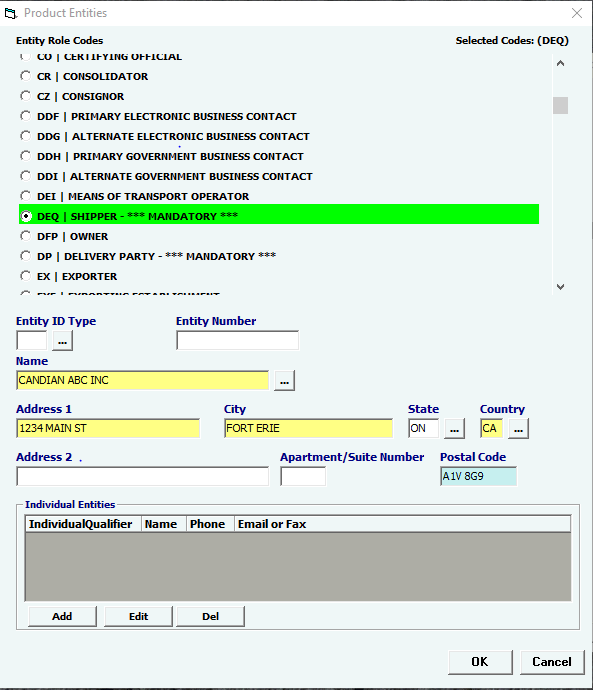
Click Add or Edit to open the product entities screen. Click Del to delete a line. |
Entity Role Codes |
Mandatory |
Select your Entity Role Codes, one at a time. Under Product Entities, enter all mandatory Entities for RADIATION. (Mandatory entites are marked ***MANDATORY*** in the picklist.)
HINT: Mandatory entities for RADIATION MF Manufacturer of goods DEQ Shipper FD1 FDA Importer (Importer of Record) DP Delivered To Party |
Entity ID Type
|
Conditional |
Select an Entity ID Type if you have an Entity ID. FDA requires Entity Name and Entity Address. Additionally, FDA prefers to use DUNS numbers for identifying the Entity; IF DUNS is not available THEN FEI |
Entity Number |
Conditional |
Enter the entity number |
Name |
Conditional |
Name of the entity. |
Address 1 |
Conditional |
Address 1 of the entity, mandatory if name is entered. |
City |
Conditional |
City of the entity, mandatory if name is entered. |
State |
Conditional |
State of the entity, mandatory if name is entered. |
Country |
Conditional |
Country of the entity, mandatory if name is entered. |
Address 2 |
Conditional |
Address 2 of the entity. |
Apartment/Suite Number |
Conditional |
Apartment/Suite Number of the entity. |
Postal Code |
Conditional |
Postal code of the entity, mandatory if name is entered. |
Add/Edit/Del |
Button |
Click Add or Edit to open the Individual Entities screen. Each Entity added must have an Individual Entity added. (This is a point of contact) This CAN be the filer’s contact info. Click Del to delete a line. |

Field/Button |
Status |
Description |
Name |
Conditional |
Name of the individual at the company. MANDATORY for all Roles |
Individual Qualifier |
Conditional |
Individual Qualifier MUST match the Entity Role Code. |
Phone Number |
Conditional |
Phone number of the individual at the company. |
Conditional |
Email of the individual at the company. |


Field/Button |
Status |
Description |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Add/Edit/Del |
Button |
Click Add or Edit to open the affirmations of compliance screen. Click Del to delete a line. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affirmation of Compliance Code | Conditional |
A
code used to affirm compliance with FDA requirements.
List of Affirmation of Compliance codes CONDITIONAL to FDA Radiation Emitting Product Message Sets
If 2877 is required:
*Annotates that additional information may be needed at time of entry in order for FDA to make a final admissibility decision.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Affirmation of Compliance Allowable Description Formats |
Conditional |
Text describing the information required by the FDA. This could include a number or a country code, etc. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Affirmation of Compliance Description |
Conditional |
Enter the Affirmation of Compliance Description |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the value lot numbers screen. Click Del to delete a line. |
Lot Number Qualifier |
Conditional
|
Note – for Lot Number Qualifier, if processing code is NSF, Lot Number Qualifier must be 3. Otherwise Lot Number Qualifier is = 1. (per PGA Supplemental Guide 2.4) |
Lot Number
|
Conditional |
The lot number that the manufacturer/ producer/grower assigned to the product |
Lot Production Start Date |
Optional |
The date when the production for the Lot started. |
Lot Production End Date |
Optional |
The date when the production for the Lot started. |
Temperature Qualifier |
Optional |
Temperature Category being reported for quality control or preservation purposes. |
Degree Type |
Optional |
F = Fahrenheit, C = Celsius , K = Kelvin |
Actual Temperature |
Optional |
Reported temperature. |
Location of Temperature Recording |
Optional |
Identifies recorded temperature is for A = product B = container C = conveyance |
PGA Line Value |
Mandatory |
The value associated with the PGA line number in whole dollars. |
PGA Unit Value |
Optional
|
The value of the lowest unit of measure |

Field/Button |
Status |
Description |
Package Quantity
|
Mandatory |
Outermost (largest=1) packages to the innermost (smallest=6) packages. The total quantity for the packaging level. |
Packaging UOM |
Mandatory |
Type of packaging / packaging level. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the containers screen. Click Del to delete a line. |
Container Number (Equipment ID) |
Optional
|
The number of the shipping container as entered in the Bill of Lading. |

Field/Button |
Status |
Description |
Arrival Status |
Mandatory |
A is the ONLY valid code for FDA: A = Anticipated arrival information. |
Anticipated Arrival date |
Mandatory
|
Date of the anticipated arrival. |
Port No. |
Optional |
Enter a valid port code. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the remarks screen. Click Del to delete a line. |
Remarks Type Code |
Optional |
A code indicating the type of remarks. FDA uses either NAM or GEN as its valid values. |
Remarks Text |
Optional |
Free form text relevant to the shipment or the commodity. |
Once you are at the bottom of the PGA screen, click "Ok" to return to the Line Items screen.

Field/Button |
Status |
Description |
Agency |
Mandatory |
Must be FDA. |
Program |
Mandatory |
RAD |
Processing Code |
Mandatory |
REP |
OK/Cancel |
Buttons |
Click OK to save the PGA Information and move on to the next screen. Cancel will cancel and go back to the PGA screen without saving. |

Field/Button |
Status |
Description |
Item Type |
Mandatory |
Defaulted to P for Product. |
| Intended Use Code | Optional |
Enter Intended Use Code *** If the Intended Use is unknown, you have the option to choose UNKN-Unknown but if not required, leave blank.
|
| Intended Use Description | Optional | Intended Use Description is optional |
| Prior Notice Number | Conditional | Enter Prior Notice Number if Prior Notice was previously submitted. |
| Product Code Qualifier | Mandatory | “FDP” (FDA Product) |
| Product Code Number | Mandatory | FDA Product Code Must be equal to 7 characters |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the source processing screen. Click Del to delete a line. |
Source Type Code |
Mandatory |
Source Type Code must be selected using the following logic. 1) IF Government Agency Program Code = FOO and Government Agency Processing Code = NSF then use 262 (Place of growth) ELSE Source Type Code can be 39 (Country of Production). IF Government Agency Program Code = FOO, THEN Country of Shipment CSH is required. IF Government Agency Program Code = FOO then Country of Entry Refusal 294 is required, if previously refused from other country(s) |
| Source Country | Mandatory | A two-letter code that identifies the country from where the product was produced, packed or shipped. |

Field/Button |
Status |
Description |
| Trade Name/Brand Name | Mandatory | Trade/Brand Name of the Radiation-Emitting Device. For example, Sony Laser scanner RM2. |
| Model | Optional | The Item Identity Number. This is the Model Number or the Serial Number based on the qualifier entered below |
| ID Number Qualifier | Optional | Code identifying the type of Item Identity Number being provided |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the product characteristics screen. Click Del to delete a line. |
| Characteristic Description | Mandatory |
Common, market or usual name or description, NOT product code description of the product. Free form description of the item. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the product entities screen. Click Del to delete a line. |
Entity Role Codes |
Mandatory |
Select your Entity Role Codes, one at a time. Under Product Entities, enter all mandatory Entities for RADIATION. (Mandatory entites are marked ***MANDATORY*** in the picklist.)
HINT: Mandatory entities for RADIATION MF Manufacturer of goods DEQ Shipper FD1 FDA Importer (Importer of Record) DP Delivered To Party |
Entity ID Type
|
Conditional |
Select an Entity ID Type if you have an Entity ID. FDA requires Entity Name and Entity Address. Additionally, FDA prefers to use DUNS numbers for identifying the Entity; IF DUNS is not available THEN FEI |
Entity Number |
Conditional |
Enter the entity number |
Name |
Conditional |
Name of the entity. |
Address 1 |
Conditional |
Address 1 of the entity, mandatory if name is entered. |
City |
Conditional |
City of the entity, mandatory if name is entered. |
State |
Conditional |
State of the entity, mandatory if name is entered. |
Country |
Conditional |
Country of the entity, mandatory if name is entered. |
Address 2 |
Conditional |
Address 2 of the entity. |
Apartment/Suite Number |
Conditional |
Apartment/Suite Number of the entity. |
Postal Code |
Conditional |
Postal code of the entity, mandatory if name is entered. |
Add/Edit/Del |
Button |
Click Add or Edit to open the Individual Entities screen. Each Entity added must have an Individual Entity added. (This is a point of contact) This CAN be the filer’s contact info. Click Del to delete a line. |

Field/Button |
Status |
Description |
Name |
Conditional |
Name of the individual at the company. MANDATORY for all Roles |
Individual Qualifier |
Conditional |
Individual Qualifier MUST match the Entity Role Code. |
Phone Number |
Conditional |
Phone number of the individual at the company. |
Conditional |
Email of the individual at the company. |


Field/Button |
Status |
Description |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Add/Edit/Del |
Button |
Click Add or Edit to open the affirmations of compliance screen. Click Del to delete a line. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affirmation of Compliance Code | Conditional |
A
code used to affirm compliance with FDA requirements.
List of Affirmation of Compliance codes CONDITIONAL to FDA Radiation Emitting Product Message Sets
If 2877 is required:
*Annotates that additional information may be needed at time of entry in order for FDA to make a final admissibility decision.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Affirmation of Compliance Allowable Description Formats |
Conditional |
Text describing the information required by the FDA. This could include a number or a country code, etc. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Affirmation of Compliance Description |
Conditional |
Enter the Affirmation of Compliance Description |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the value lot numbers screen. Click Del to delete a line. |
Lot Number Qualifier |
Conditional
|
Note – for Lot Number Qualifier, if processing code is NSF, Lot Number Qualifier must be 3. Otherwise Lot Number Qualifier is = 1. (per PGA Supplemental Guide 2.4) |
Lot Number
|
Conditional |
The lot number that the manufacturer/ producer/grower assigned to the product |
Lot Production Start Date |
Optional |
The date when the production for the Lot started. |
Lot Production End Date |
Optional |
The date when the production for the Lot started. |
Temperature Qualifier |
Optional |
Temperature Category being reported for quality control or preservation purposes. |
Degree Type |
Optional |
F = Fahrenheit, C = Celsius , K = Kelvin |
Actual Temperature |
Optional |
Reported temperature. |
Location of Temperature Recording |
Optional |
Identifies recorded temperature is for A = product B = container C = conveyance |
PGA Line Value |
Mandatory |
The value associated with the PGA line number in whole dollars. |
PGA Unit Value |
Optional
|
The value of the lowest unit of measure |

Field/Button |
Status |
Description |
Package Quantity
|
Mandatory |
Outermost (largest=1) packages to the innermost (smallest=6) packages. The total quantity for the packaging level. |
Packaging UOM |
Mandatory |
Type of packaging / packaging level. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the containers screen. Click Del to delete a line. |
Container Number (Equipment ID) |
Optional
|
The number of the shipping container as entered in the Bill of Lading. |

Field/Button |
Status |
Description |
Arrival Status |
Mandatory |
A is the ONLY valid code for FDA: A = Anticipated arrival information. |
Anticipated Arrival date |
Mandatory
|
Date of the anticipated arrival. |
Port No. |
Optional |
Enter a valid port code. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the remarks screen. Click Del to delete a line. |
Remarks Type Code |
Optional |
A code indicating the type of remarks. FDA uses either NAM or GEN as its valid values. |
Remarks Text |
Optional |
Free form text relevant to the shipment or the commodity. |
Once you are at the bottom of the PGA screen, click "Ok" to return to the Line Items screen.
Field/Button |
Status |
Description |
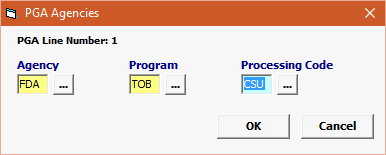
Agency |
Mandatory |
Must be FDA. |
Program |
Mandatory |
TOB |
Processing Code |
Mandatory |
· Enter the appropriate Processing Code (CSU, FFM, INV) |
OK/Cancel |
Buttons |
Click OK to save the PGA Information and move on to the next screen. Cancel will cancel and go back to the PGA screen without saving. |

Field/Button |
Status |
Description |
Item Type |
Mandatory |
Defaulted to P for Product. |
| Intended Use Code | Conditional |
Enter Intended Use Code *** If the Intended Use is unknown, you have the option to choose UNKN-Unknown but if not required, leave blank.
|
| Intended Use Description | Optional | Intended Use Description is optional |
| Product Code Qualifier | Mandatory | “FDP” (FDA Product) |
| Product Code Number | Mandatory | FDA Product Code Must be equal to 7 characters |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the source processing screen. Click Del to delete a line. |
Source Type Code |
Mandatory |
For Tobacco, Source Type Code 39 (Country of Production) is required. Other Source Type Codes, 262 (Place of Growth) or HRV (Harvested) or 30 (Country of Source) may be entered, if available. There would be at least one PG06 with source type code of 39. More PG06 records may be repeated for the optional Source Type Codes, 262, HRV or 30.. Additionally, if previously refused, then trade would also provide another PG06 with source type code 294 (Country of Refusal). |
| Source Country | Mandatory | Country of production or source is required for Tobacco. |
Field/Button |
Status |
Description |
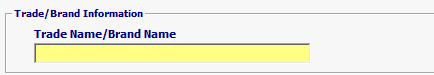
| Trade Name/Brand Name | Conditional | If Government Agency Processing Code is INV (Investigational) or CSU (Consumer Use) then trade or brand name is mandatory. If Government Agency Processing Code is FFM (For Further Manufacturing), then trade name/brand name is optional. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the product characteristics screen. Click Del to delete a line. |
| Characteristic Description | Mandatory |
Free form invoice description, NOT product code description. This field will capture such information as length, color, and pack count. Under 21 1140.16(b), cigarette packages are required to contain a minimum of 20 cigarettes. |

Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
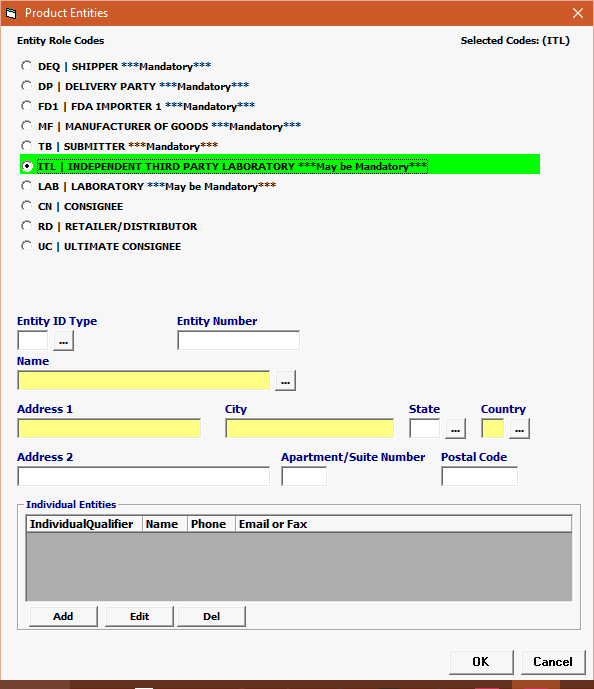
Click Add or Edit to open the product entities screen. Click Del to delete a line. |
Entity Role Codes |
Mandatory |
Select your Entity Role Codes, one at a time.Under Product Entities, enter all mandatory Entities for TOBACCO. (Mandatory entites are marked ***MANDATORY*** in the picklist.) HINT: Mandatory entities for TOBACCO MF Manufacturer of goods DEQ Shipper FD1 FDA Importer (Importer of Record) TB Submitter DP Delivered To Party |
Entity ID Type
|
Conditional |
Select an Entity ID Type if you have an Entity ID. FDA requires Entity Name and Entity Address. Additionally, FDA prefers to use DUNS numbers for identifying the Entity; IF DUNS is not available THEN FEI |
Entity Number |
Conditional |
Enter the entity number |
Name |
Conditional |
Name of the entity. |
Address 1 |
Conditional |
Address 1 of the entity, mandatory if name is entered. |
City |
Conditional |
City of the entity, mandatory if name is entered. |
State |
Conditional |
State of the entity, mandatory if name is entered. |
Country |
Conditional |
Country of the entity, mandatory if name is entered. |
Address 2 |
Conditional |
Address 2 of the entity. |
Apartment/Suite Number |
Conditional |
Apartment/Suite Number of the entity. |
Postal Code |
Conditional |
Postal code of the entity, mandatory if name is entered. |
Add/Edit/Del |
Button |
Click Add or Edit to open the Individual Entities screen. Each Entity added must have an Individual Entity added. (This is a point of contact) This CAN be the filer’s contact info. Click Del to delete a line. |

Field/Button |
Status |
Description |
Name |
Conditional |
Name of the individual at the company. MANDATORY for all Roles |
Individual Qualifier |
Conditional |
Individual Qualifier MUST match the Entity Role Code. |
Phone Number |
Conditional |
Phone number of the individual at the company. |
Conditional |
Email of the individual at the company. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the affirmations of compliance screen. Click Del to delete a line. |
| Affirmation of Compliance Code | Conditional |
A code used to affirm compliance with FDA requirements. |
Affirmation of Compliance Allowable Description Formats |
Conditional |
Text describing the information required by the FDA. This could include a number or a country code, etc. |
Affirmation of Compliance Description |
Conditional |
Enter the Affirmation of Compliance Description |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the value lot numbers screen. Click Del to delete a line. |
Lot Number Qualifier |
Optional |
Includes Lots and/or Batches IF Government Agency Program Code = TOB THEN Lot Number Qualifier = 3 |
Lot Number
|
Optional |
The lot number that the manufacturer/ producer/grower assigned to the product |
Lot Production Start Date |
Optional |
The date when the production for the Lot started. |
Lot Production End Date |
Optional |
The date when the production for the Lot started. |
Temperature Qualifier |
Optional |
Temperature Category being reported for quality control or preservation purposes. |
Degree Type |
Optional |
F = Fahrenheit, C = Celsius , K = Kelvin |
Actual Temperature |
Optional |
Reported temperature. |
Location of Temperature Recording |
Optional |
Identifies recorded temperature is for A = product B = container C = conveyance |
PGA Line Value |
Mandatory |
The value associated with the PGA line number in whole dollars. Line Value is needed for enforcement of User Fee Regulations. Failure to pay user fees makes a product adulterated under Section 902 of the FD&C Act in accordance with Section 919. |
PGA Unit Value |
Mandatory |
The value of the lowest unit of measure |

Field/Button |
Status |
Description |
Package Quantity
|
Mandatory |
Outermost (largest=1) packages to the innermost (smallest=6) packages. The total quantity for the packaging level. |
Packaging UOM |
Mandatory |
Type of packaging / packaging level. |

Field/Button |
Status |
Description |
Arrival Status |
Mandatory |
A is the ONLY valid code for FDA: A = Anticipated arrival information. |
Anticipated Arrival date |
Mandatory
|
Date of the anticipated arrival. |
Port No. |
Optional |
Enter a valid port code. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the remarks screen. Click Del to delete a line. |
Remarks Type Code |
Optional |
A code indicating the type of remarks. FDA uses either NAM or GEN as its valid values. |
Remarks Text |
Optional |
Free form text relevant to the shipment or the commodity. |
Once you are at the bottom of the PGA screen, click "Ok" to return to the Line Items screen.
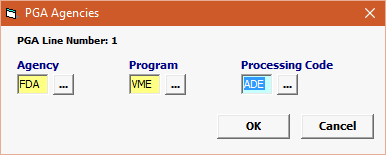
Field/Button |
Status |
Description |
Agency |
Mandatory |
Must be FDA. |
Program |
Mandatory |
VME |
Processing Code |
Mandatory |
Enter the appropriate Processing Code (ADE or ADR) |
OK/Cancel |
Buttons |
Click OK to save the PGA Information and move on to the next screen. Cancel will cancel and go back to the PGA screen without saving. |

Field/Button |
Status |
Description |
Item Type |
Mandatory |
Defaulted to P for Product. |
| Intended Use Code | Conditional |
Enter Intended Use Code *** If the Intended Use is unknown, you have the option to choose UNKN-Unknown but if not required, leave blank.
|
| Intended Use Description | Optional | Intended Use Description is optional |
| Product Code Qualifier | Mandatory | “FDP” (FDA Product) |
| Product Code Number | Mandatory | FDA Product Code Must be equal to 7 characters |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the constituent elements screen. Click Del to delete a line. |
| Active Ingredient | Mandatory |
Active ingredient = “Y” if yes, blank if no. |
| Name of Active Ingredient | Mandatory
|
IF Government Agency Program Code = VME AND Government Agency Processing Code = ADR THEN Constituent Active Ingredient Qualifier and Name of the Constituent Element are Mandatory. |
| Percent of Active Ingredient | Mandatory
|
Either the quantity and Unit of Measurement should be entered OR the Percent Constituent Element should be entered. |
| Quantity of Active Ingredient | Mandatory
|
Either the quantity and Unit of Measurement should be entered OR the Percent Constituent Element should be entered. |
| Unit of Measure | Mandatory
|
Enter the unit of measure. |
Field/Button |
Status |
Description |
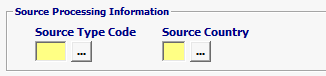
Source Type Code |
Mandatory |
Mandatory valid values are 30 (Country of Source) or 39 (Country of Production) is MANDATORY. 294 (Country of Refusal) if previously refused |
| Source Country | Mandatory | Country of production or source is required for Animal Drugs |

Field/Button |
Status |
Description |
| Trade Name/Brand Name | Conditional | If Government Agency Program Code = ‘VME’ the Trade/Brand Name of the Animal Drug is MANDATORY. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the product characteristics screen. Click Del to delete a line. |
| Characteristic Description | Mandatory |
Free form description, NOT product code description, of the item, either to supplement the above data elements or in place of the above. |

Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
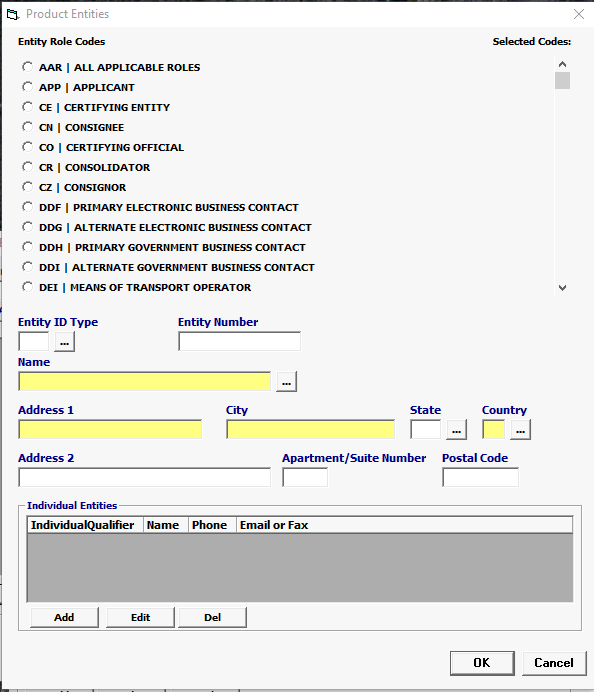
Click Add or Edit to open the product entities screen. Click Del to delete a line. |
Entity Role Codes |
Mandatory |
Select your Entity Role Codes, one at a time. Under Product Entities, enter all mandatory Entities for VETERINARY MEDICAL. (Mandatory entites are marked ***MANDATORY*** in the picklist.) HINT: Mandatory entities for VETERINARY MEDICAL MF Manufacturer of goods DEQ Shipper FD1 FDA Importer (Importer of Record) DP Delivered To Party |
Entity ID Type
|
Conditional |
Select an Entity ID Type if you have an Entity ID. FDA requires Entity Name and Entity Address. Additionally, FDA prefers to use DUNS numbers for identifying the Entity; IF DUNS is not available THEN FEI |
Entity Number |
Conditional |
Enter the entity number |
Name |
Conditional |
Name of the entity. |
Address 1 |
Conditional |
Address 1 of the entity, mandatory if name is entered. |
City |
Conditional |
City of the entity, mandatory if name is entered. |
State |
Conditional |
State of the entity, mandatory if name is entered. |
Country |
Conditional |
Country of the entity, mandatory if name is entered. |
Address 2 |
Conditional |
Address 2 of the entity. |
Apartment/Suite Number |
Conditional |
Apartment/Suite Number of the entity. |
Postal Code |
Conditional |
Postal code of the entity, mandatory if name is entered. |
Add/Edit/Del |
Button |
Click Add or Edit to open the Individual Entities screen. Each Entity added must have an Individual Entity added. (This is a point of contact) This CAN be the filer’s contact info. Click Del to delete a line. |

Field/Button |
Status |
Description |
Name |
Conditional |
Name of the individual at the company. MANDATORY for all Roles |
Individual Qualifier |
Conditional |
Individual Qualifier MUST match the Entity Role Code. |
Phone Number |
Conditional |
Phone number of the individual at the company. |
Conditional |
Email of the individual at the company. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the affirmations of compliance screen. Click Del to delete a line. |
| Affirmation of Compliance Code | Conditional |
A code used to affirm compliance with FDA requirements. There must be at least one PG23 record with the AoC code of REG. |
Affirmation of Compliance Description |
Conditional |
Enter the Affirmation of Compliance Description |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the value lot numbers screen. Click Del to delete a line. |
Lot Number Qualifier |
Optional |
Includes Lots and/or Batches IF Government Agency Program Code = TOB THEN Lot Number Qualifier = 3 |
Lot Number
|
Optional |
The lot number that the manufacturer/ producer/grower assigned to the product |
Lot Production Start Date |
Optional |
The date when the production for the Lot started. |
Lot Production End Date |
Optional |
The date when the production for the Lot started. |
Temperature Qualifier |
Optional |
Temperature Category being reported for quality control or preservation purposes. |
Degree Type |
Optional |
F = Fahrenheit, C = Celsius , K = Kelvin |
Actual Temperature |
Optional |
Reported temperature. |
Location of Temperature Recording |
Optional |
Identifies recorded temperature is for A = product B = container C = conveyance |
PGA Line Value |
Mandatory |
The value associated with the PGA line number in whole dollars. |
PGA Unit Value |
Optional |
The value of the lowest unit of measure |

Field/Button |
Status |
Description |
Package Quantity
|
Mandatory |
Outermost (largest=1) packages to the innermost (smallest=6) packages. The total quantity for the packaging level. |
Packaging UOM |
Mandatory |
Type of packaging / packaging level. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the containers screen. Click Del to delete a line. |
Container Number (Equipment ID) |
Optional
|
The number of the shipping container as entered in the Bill of Lading. |

Field/Button |
Status |
Description |
Arrival Status |
Mandatory |
A is the ONLY valid code for FDA: A = Anticipated arrival information. |
Anticipated Arrival date |
Mandatory
|
Date of the anticipated arrival. |
Port No. |
Optional |
Enter a valid port code. |


Field/Button |
Status |
Description |
Add/Edit/Del |
Button |
Click Add or Edit to open the remarks screen. Click Del to delete a line. |
Remarks Type Code |
Optional |
A code indicating the type of remarks. FDA uses either NAM or GEN as its valid values. |
Remarks Text |
Optional |
Free form text relevant to the shipment or the commodity. |
Once you are at the bottom of the PGA screen, click "Ok" to return to the Line Items screen.